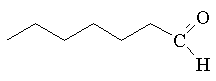
Chapter 16 Homework Solutions
17 Aldehydes can be oxidized to carboxlyic acids; ketones cannot. The Tollens test and Benedicts test take advantage of this. Both tests involve reagents which react with aldehydes to produce a visible product. No reaction occurs with ketones.
18 See the solution in the text book (page A-30).
21 The cyclic structure is more stable, so most of the molecules exist in this form.
24 a. aldehyde b. amide c. no carbonyl d. no carbonyl e. carboxylic acid f. ketone
26 This problem was accidentally left off the list

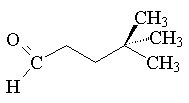
d.) 3-hydroxy-2,2,4-trimethylpentanal: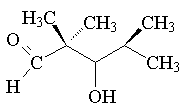
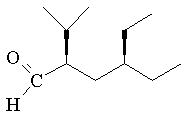
30 a,b. The names 1-butanone and 4-butanone are incorrect because the ketone group cannot appear at the end of the alkyl chain.
c. Should be 2-butanone.
32
A hemiacetal is formed when an aldehyde reacts with an alcohol in a 1:1 ratio. An acetal has two OR groups attached to a carbon, while a hemiacetal has an OH and an OR.
36 See the answers in the text.
a.)
b.)
c.)
38 See the answers in the text book.
40
52 Vanillin is an aromatic compound, so the solvent should be organic. The polar functional groups (hydroxyl and methoxy) suggest a polar solvent. Ethanol meets both requirements while being non-toxic.
60
Ketones are far more resistant to oxidation than are aldehydes. When the aldehyde is oxidized (in air), a generally unattractive carboxylic acid is the result.
70
Alcohols form stronger H-bonds than amines, so 1-butanol would have a higher boiling point than butylamine. Butanal does not H-bond to itself, so its bp would be the lowest.